About MaskIT
MaskIt was created by health care providers to generate ways to protect the health care team. Born out of the COVID-19 crisis, the priority was to create a source protection device offering increased security to the provider, those in the exam room, and the room itself. From inception, we have focused on providing premium products to add safety and security for all involved in patient care.
Secure Common Spaces
Many infectious diseases such as Covid-19, spread from the mouth and nose. Ear, Nose and Throat Doctors utilize endoscopes in the office as a critical tool for evaluating patients. Although the endoscope itself has never been proven to be an aerosol producing procedure, the events that occur during the scope have the potential to produce aerosols. These procedures are typically done in confined office exam rooms. Patients can sneeze, cough, or gag, events that have been shown to increase infectious spread. This converts a procedure that is non aerosolizing into one that is potentially explosive with infectious material.

PPE options when treating infected or potentially infected patients are many. Universal precautions and source protection are the first and strongest barriers. It has been shown with many diseases including TB, HIV, STDs and now Covid-19 that universal precautions using PPE and source protection improve safety and reduce spread. Treating respiratory diseases such as Covid-19 must include source protection as a method to inhibit large volumes of infectious droplets from exposing and contaminating closed spaces.
There are a variety of options to help protect providers including gowns, face shields, PAPR, air filters, negative pressure rooms, and closing rooms to allow particles to come to rest. Although these are reasonable options, nothing is being done to isolate the source. Testing patients before each visit relies on the validity of the test and is expensive to the health care system.
Why Diagnostic Guardian™?
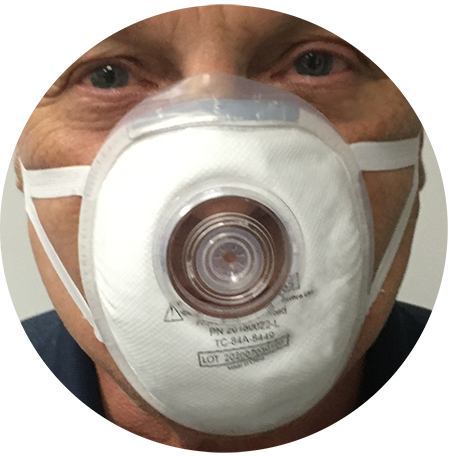
The Diagnostic Guardian™ family of products was created by using a base N95 mask and securing a biomedical seal into it, securing our common spaces. The FDA has authorized the Diagnostic and Therapeutic Guardian Mask family under the Emergency Utilization Act (EUA) for use as a source control. As such it has not been formally evaluated by the FDA. The Therapeutic and Guardian Mask family are not to be used in any other application other than a source protection device to assist in reducing spread of infectious material during endoscopy.